
The Basics
COVID-19 where did it come from and where is it going? To start let’s discuss the history of the virus. SARS-CoV-2 or COVID-19 is a single-stranded positive-sense RNA virus that is part of a zoonotic Coronaviridae family and is most genetically similar to SARS-CoV-1 (Bahchechi, M.). This means that the virus currently wreaking havoc on the world evolved from a similar variant of a similar disease in a closely related set of diseases. This set of diseases are considered zoonotic which means that they originate first in animals before entering the human population. The genetic material of this virus is RNA, typical cells use DNA as their genetic material. Viruses are a type of pathogen that require hosts to survive and replicate, therefore they are not considered to be alive. But if viruses are not alive, how can they evolve?
Similar to all living organisms, viruses are constantly evolving, mutations or changes in their genetic material can cause variations in the structure of the virus. The random force of evolution (mutations) is acting on viruses therefore predictable forces like natural selection also affect their evolution. Like all organisms, viruses are in a constant arms race against other pathogens and the hosts they inhabit. According to the red queen hypothesis, pathogens and hosts are constantly evolving to overcome challenges presented by their interactions (sironi, M.). What are these host-pathogen interactions, and how do they influence the evolution of disease?

The Red Queen Hypothesis
hosts are under pressure to evolve resistance to pathogens while pathogens strive to develop countermeasures to evade host surveillance and to achieve a successful infection
(sironi, M.)
Evolutionary Trends
Positive and negative interactions between organisms and their environment act as selective forces influencing particular characteristics of species propelling evolution. Advantageous characteristics are selected for and occur more frequently in subsequent populations. Because we are focusing on human disease, we are going to look more at negative pathogen-host interactions that drive their co-evolution.
Diseases negatively affect their hosts causing an overall decrease in survival, while advantages within the host immune system negatively affect the invading pathogen. Based on these interactions there are predictable trends with the evolution of both a host’s immune system and the pathogen. Diseases are characterized by their virulence or ability to cause severe illness. Extremely virulent diseases that interact with highly susceptible hosts often show a reduction in virulence over time, increasing the spread of disease by prolonging the life of their host. On the other hand, as hosts become less susceptible to illness the virulence of the disease often increases, creating more dangerous strains and variants for those who remain highly susceptible (Read, A.). A prime example is of this concept can be seen through subsequent outbreaks of cholera. Originating in India for decades the bacteria that caused cholera was of low virulence and seemed to spread slowly and sporadically. It was not until the 1845 outbreak in London where a random mutation upped the virulence and outcompeted previous strains. Often blamed on urbanization the proximity of susceptible hosts allows for a fast-spreading bacterium to become more prevalent and caused more damage (McMillen, C.).
Factors other than host immunity can also influence the evolution of diseases, societal changes have a large impact on disease evolution and introduce multiple selective factors that encourage the evolution of high virulence. Changes in the way we live including repurposing land for agriculture, the urbanization of cities, and poor health populations all provide risks for the emergence of new disease (Reid, S.). These changes can increase the chance that humans cross with animals and are introduced to new diseases from those animals. These zoonotic diseases originate in animals then spill over into the human population causing outbreaks. Humans act as new vulnerable hosts that do not possess the high immunity already present in the animal population.
This form of disease outbreak references emerging diseases and are new illnesses in human populations. Whereas re-emerging diseases more often referenced in evolutionary history are upticks in diseases already in the population. This type of transmission has been recognized throughout history causing outbreaks of a vast variety of diseases including, the Spanish flu in 1918, the swine flu in 2009, the Ebola outbreak in 2014, and the Zika outbreak in 2015 (Huremovic, D.). The Zika virus originated in rhesus monkeys first emerged in human populations in 2007 transmitted by mosquitoes did not become a full epidemic until its re-emergence by a second transmission event in Brazil in 2015 (Huremovic, D.). The evolved differences in the virus across this period resulted in an increased virulence that led to the outbreak.
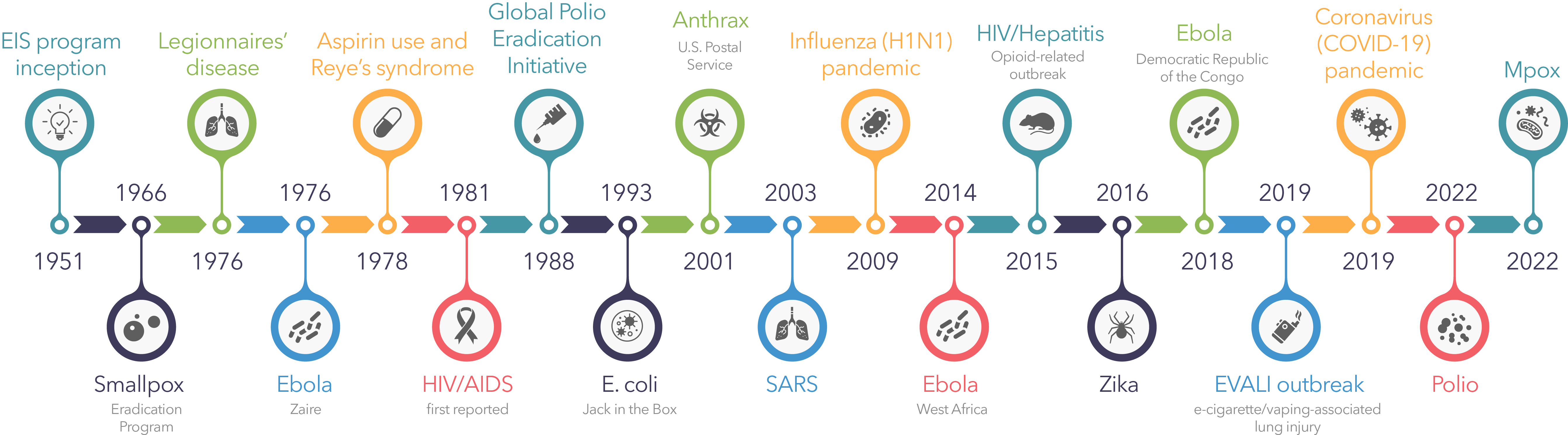
Lastly, the introduction of antibiotic drugs and vaccines act as additional selective factors for disease evolution. Antibiotics are used after infection occurs and target specific components to kill bacteria. This means that if not all bacteria are susceptible to the antibiotic used resistance spreads rapidly and the disease persists. The selective pressure that antibiotics introduce is extreme and can result in resistant strains spreading quickly. A good example of this is the continued emergence of antibiotic-resistant tuberculosis strains, first appearing in Kenya in 1950-1960 (McMillen, C.). Vaccinations are used to prevent infection and the spread of disease. Because vaccinations prevent spread it limits the chances for new variants to arise and have little effect on the evolution of slow mutating viruses (Soquel, C.). However, fast mutating viruses like the flu show different trends. The rapidly mutating nature of the negative-sense single-stranded RNA genome allows for variants of two strains to consistently circulate in the population as the seasonal flu (Petrova, V.). In this circumstance, vaccinations act as a selective pressure against the variant it targets since multiple strains present in the population outcompete each other at different rates every year.
Like all diseases COVID-19 is already evolving, several new variants have already been identified abroad and in the united states. Most variants do not persist, because they have no advantageous mutations. However, those that do can lead to more problems as the pandemic progresses including, higher virulence, rapid spreading, and a possibility for ineffective vaccines. According to the CDC, the current persistent variants do not outnumber the original variant and can still be recognized by the antibodies produced from available vaccinations (CDC).
Work Cited
“About Variants of the Virus that Causes COVID-19.” Centers for disease control and prevention. 2, April. 2021. https://www.cdc.gov/coronavirus/2019-ncov/transmission/variant.html#:~:text=Multiple%20variants%20of%20the%20virus,spikes%20on%20their%20surfaces, Accessed 30 April 20201
Baghchechi, M., Jaipaul, N., Jacob E. S. “The rise and evolution of COVID-19.” Science Direct, Vol.6, no. 4, 2020 248-254. https://doi.org/10.1016/j.ijwd.2020.06.006
Huremovic D. “Brief History of Pandemics (Pandemics Throughout History).” Psychiatry of Pandemics. 2019 pp. 7-35. doi: 10.1007/978-3-030-15346-5_2
McMillen, W. Christian. Pandemics, A very short introduction. Oxford press, 2016.
Petrova. N. V., Russell, A. C. “The evolution of seasonal influenza viruses.” Nature Reviews Microbiology. 2017 pp. 47-60. https://doi.org/10.1038/nrmicro.2017.118, Accessed 30 April 20201
Read, F. A., Kerr, J. P. “Protection at a price”. The Scientist. pp. 41-47.
Reid, S. “Disease evolution: how new illness emerge when we change how we live”. The Conversation. 28, April. 2016. https://theconversation.com/disease-evolution-how-new-illnesses-emerge-when-we-change-how-we-live-54570, Accessed 30 April 20201
Sironi, M., Cagliani, R., Forni, D. et al. “Evolutionary insights into host–pathogen interactions from mammalian sequence data.” Nature Reviews Genetics,2015 pp. 224–236. https://doi.org/10.1038/nrg3905
Souque, C., Plessis, L. “Why resistance is common in antibiotics, but rare in vaccines.” The Conversation. 11, January. 2021 https://theconversation.com/why-resistance-is-common-in-antibiotics-but-rare-in-vaccines-152647, Accessed 30 April 20201

While reading this passage, I forgot I was reading a college student’s paper. It seemed as though I was reading an article from a science magazine or from an infromation flyer about COVID-19 that I would find in the waiting room at the doctor’s office. This entire website is very impressive, something I will forward to my collegues, friends and family!